
离岸价格
Get Latest Price|
10000 Minimum Order
国:
China
モデル番号:
H1N1
离岸价格:
ロケーション:
China Mainland
最低注文量の価格:
-
最低注文量:
10000
パッケージの詳細:
-
納期:
5DAYS
供給能力:
100000
支払いタイプ:
-
製品グループ :
-
連絡先担当者 Mr. Davy W
ZMC BUILDING,,101-2 N ZHONGSHAN RD, HANGZHOU, Other
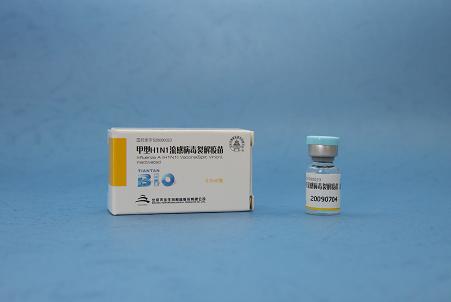
Dosage form: Injections Validity: 1 year Packing: O.5ml in vial or syringe in a small carton box 0.*5 ml in vial or sryinge in a small carton box The vaccine production starts with the inoculation of the virus into the allantoic fluid of chicken embryos, where it multiplies. After a certain period of incubation the virus is harvested, inactivated and purified using different downstream processing techniques. The purified whole virus is then split, using a detergent and further purified to yield the so-called monobulk. These production steps are applied separately for each of the three vaccine components. In the last step, the components are blended in the correct proportion, and the mixture is filled into syringes or vials of 0.5 ml for adults and 0.*5 ml for children. A syringe or vial contains one dose of trivalent influenza vaccine in the required amount of *5 μg HA per strain for adults and 7.5 ug HA for children. Finally, the vaccine quality is checked and then marketed after approval by the national regulatory authority (SFDA). The product is in compliance with the EU standard. This influenza vaccine is an inactivated split vaccine, produced on embryonated chicken eggs. The vaccine is manufactured according to the seasonal WHO recommendation for Northern and Southern Hemisphere. The vaccine&#**9;s quality level is comparable to internationally available vaccines.
| 国: | China |
| モデル番号: | H1N1 |
| 离岸价格: | Get Latest Price |
| ロケーション: | China Mainland |
| 最低注文量の価格: | - |
| 最低注文量: | 10000 |
| パッケージの詳細: | - |
| 納期: | 5DAYS |
| 供給能力: | 100000 |
| 支払いタイプ: | - |
| 製品グループ : | - |