



离岸价格
Get Latest Price( Negotiable )
|50000 Piece Minimum Order
国:
Others1
モデル番号:
According to Customer Specification
离岸价格:
( Negotiable ) Get Latest Price
ロケーション:
Others1
最低注文量の価格:
-
最低注文量:
50000 Piece
パッケージの詳細:
as per attach photo or brochure
納期:
2-3 weeks
供給能力:
100000 Piece per Week
支払いタイプ:
T/T
製品グループ :
連絡先担当者 Mr. Sam
Room 302C, No.718, Blv San Yuan Li, Bai Yuan District,, Changping, Guangdong
| 国: | Others1 |
| モデル番号: | According to Customer Specification |
| 离岸价格: | ( Negotiable ) Get Latest Price |
| ロケーション: | Others1 |
| 最低注文量の価格: | - |
| 最低注文量: | 50000 Piece |
| パッケージの詳細: | as per attach photo or brochure |
| 納期: | 2-3 weeks |
| 供給能力: | 100000 Piece per Week |
| 支払いタイプ: | T/T |
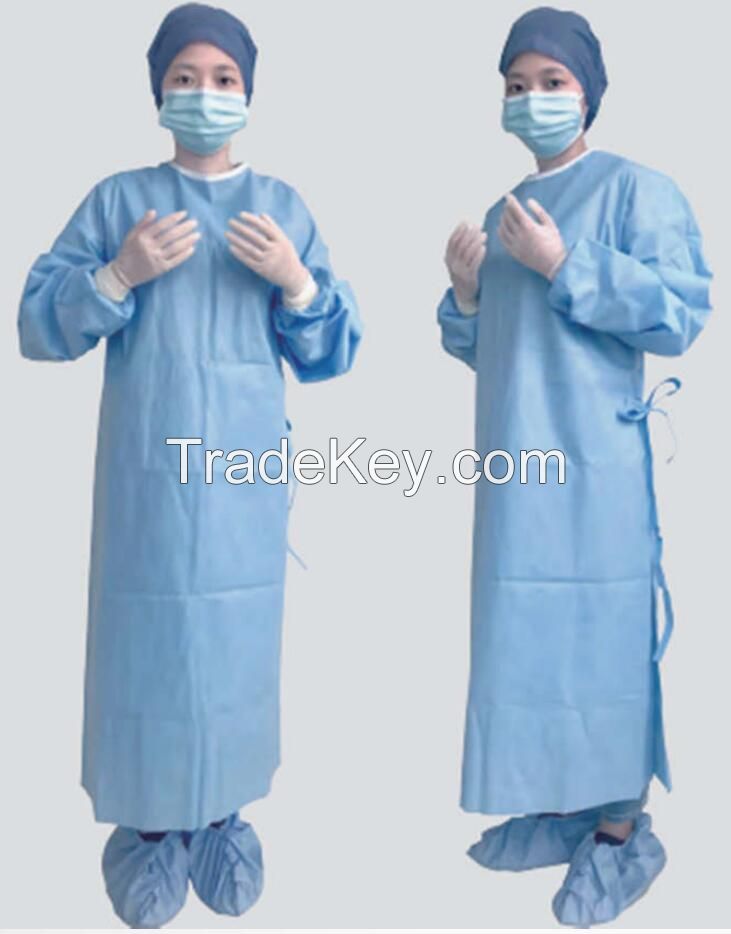
| 製品グループ : | surgical suit |