Hangzhou,Zhejiang,China China
 "Healthy life style Foot Soak moxa pie with Ginger formula and safflower formula "
"Healthy life style Foot Soak moxa pie with Ginger formula and safflower formula "
 "Wormwood health cushion with 3 years chen Qi chun county moxa punk "
"Wormwood health cushion with 3 years chen Qi chun county moxa punk "
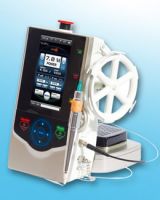 10W Dental Laser System
10W Dental Laser System
 12 channel ECG (manufacturer)
12 channel ECG (manufacturer)
 15W EVLT Diode Laser System
15W EVLT Diode Laser System
 3 Channel ECG (manufacturer)
3 Channel ECG (manufacturer)
 Acupoint Therapy Plaster Mugwort San Fu patch for winter disease cured in summer complementary therapy
Acupoint Therapy Plaster Mugwort San Fu patch for winter disease cured in summer complementary therapy
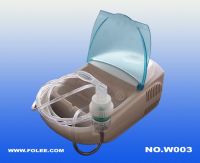 Air-compressing Nebulizer
Air-compressing Nebulizer
 Ampoule Filling And Sealing Machine
Ampoule Filling And Sealing Machine
 AS|NZS P1 MASK
AS|NZS P1 MASK
Established in ***2, Hangzhou Gongkang Medical Technology Co., Ltd. was a plastic accessories provider for domestic medical device manufacturers. After *3 years of effort, the company introduced the Singleuse Containers for Human Venous Blood Specimen Collection Tube production project in ***1. Gong Kang hired a professional team with more than *0 years of experience in this field. Prior to joining Gong Kang, this team provided technical support, quality control and production management for listed companies. With a good starting point, strict standard, and heavy investment, the company now has a Class *0,**0 clean room laboratory, a Class **0,**0 clean room production purification plant and the latest overseas and domestic production equipment and testing equipment at home and abroad. The company obtained the sterile medical devices GMP certification first in ***3, and successively acquired medical devices production certificate and product registration certificate. Meanwhile, the company also passed ISO ****5 and obtained CE Marking (TUV SUD).
今すぐお問い合わせください| ビジネスタイプ | Others |
| ウェブサイト | N/A |
| 設立年 | 1992 |
| 従業員数 | 100+ |
| 主要市場 | Worldwide |
| 企業製品/サービス | blood collection tube |
| 工場所在地 | N/A |
| 工場サイズ | N/A |
| 生産ライン数 | 0 |
| 年間総購買量の合計 | N/A |
| 研究開発スタッフの人数 | N/A |
| 品質管理 | N/A |
| 登記書 | N/A |
| 契約製造 | N/A |
| 登録資本金 | N/A |
| 所有権タイプ | N/A |
| 法定代表者/CEO | N/A |
| エクスポートパーセンテージ | N/A |
| 年間売上高合計 | N/A |
| QCスタッフの人数 | N/A |
| 連絡先担当者 | Amy |
| 会社 | Hangzhou Gongkang Medical technology Co., Ltd |
| 電話 | ******** |
| Mobile | ******** |
| ファックス | ******** |